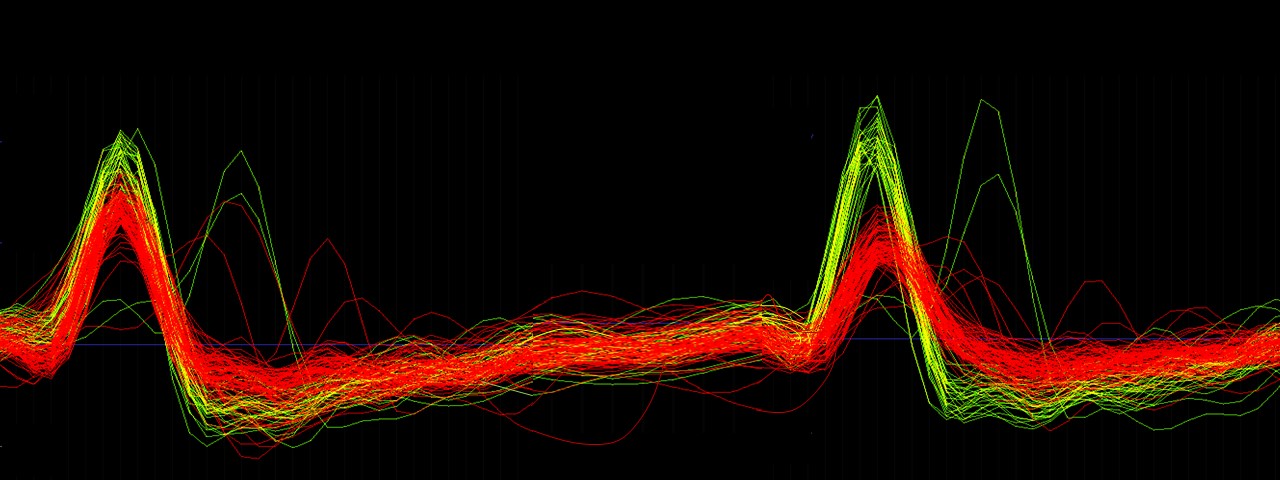
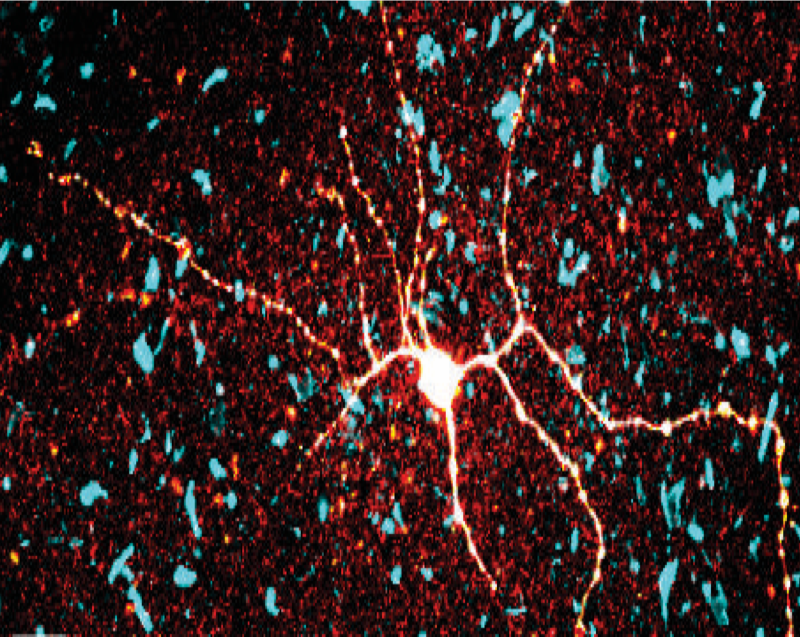
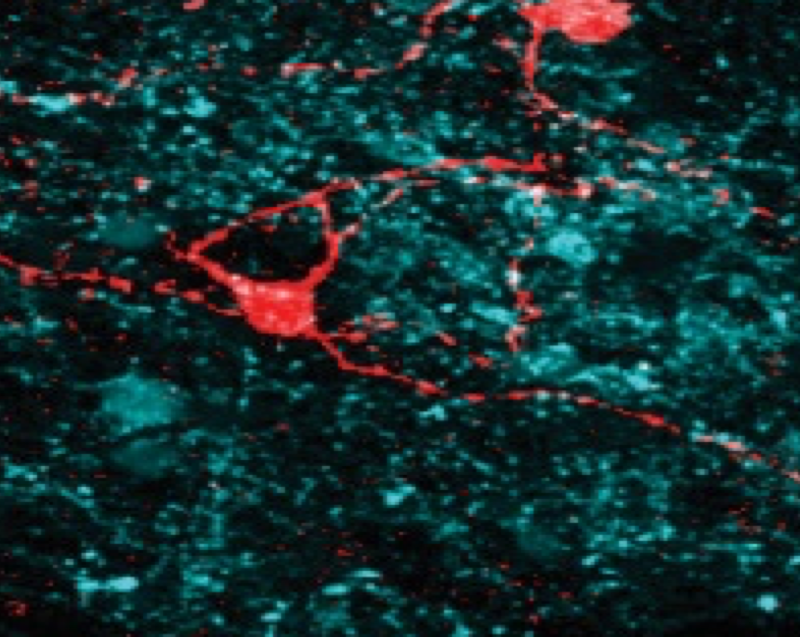
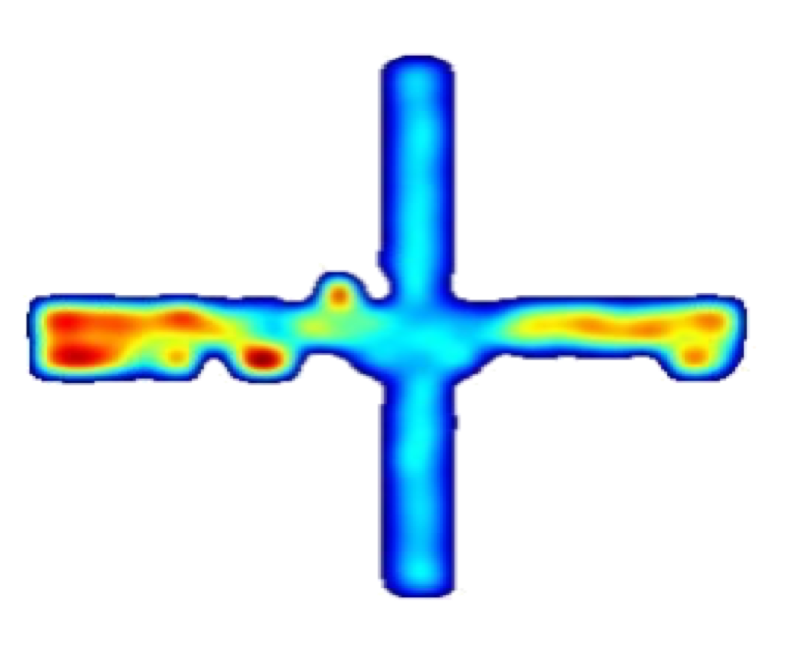
Research Interests
Changes in behavioral states let us rapidly adapt and react to opportunities and dangers in an ever-changing environment, and are thus critical to ensure survival. The behavioral state we are most interested in is the high anxiety state, which allows animals to assess risks while minimizing exposure to potential threats. The anxious state involves many adaptations, including avoidance of danger, increases in heart rate and changes in stress-related hormones in the blood stream. Furthermore, anxiety levels modulate changes in other behaviors as well, such as eating and sleeping. Although it is known that high anxiety is accompanied by multiple manifestations, little is known about the specific neural circuits that control each of symptom. How does anxiety affect eating or aggression? Are physiological and behavioral symptoms of anxiety modulated by the same circuits?
Our goal is to answer questions such as these by dissecting how interactions between different brain regions orchestrate the numerous symptoms of the anxious state using mice as a model organism. To achieve an integrated view of these phenomena we use a wide range of techniques, including electrophysiology, behavior, fiber photometry and optogenetics. The combination of these powerful techniques allows us to monitor and manipulate neural activity at both the microcircuit and long-range connection levels, providing causal relationships between neural activity and specific behaviors in mice.
Highlights of Previous Discoveries
Press on the + next to the title for a summary of the listed publications
Characterization of ventromedial hypothalamus activity during exposure to innate and conditioned threats
Summary
In the face of imminent predatory danger, animals quickly detect the threat and mobilize key survival defensive actions, such as escape and freezing. The dorsomedial portion of the ventromedial hypothalamus (VMH) is a central node in innate and conditioned predator-induced defensive behaviors. Prior studies have shown that activity of steroidogenic factor 1 (sf1)-expressing VMH cells is necessary for predator-induced such defensive behaviors. We show that these neurons are activated by threat proximity and that they encode future occurrence of escape. Our data also show that VMH cells encoded proximity of an innate predatory threat but not a fear-conditioned shock grid. Furthermore, chemogenetic activation of the VMH increases avoidance of innate threats, such as open spaces and a live predator. These data show that different circuits in the brain can mediate fear induced by a innate (a live predator) or learned (a shock grid) threats.
Characterization of ventromedial hypothalamus activity during exposure to innate and conditioned threats. Eur J Neurosci. 2023 Apr;57(7):1053-1067. [pdf]
Orchestration of innate and conditioned defensive actions by the periaqueductal gray
Summary
The midbrain periaqueductal gray (PAG) has been recognized for decades as having a central role in threat-induced actions. Here, we discuss both classic and modern studies reporting on how PAG-centered circuits influence innate as well as learned defensive actions in rodents and humans. Though early discoveries emphasized the PAG’s role in rapid induction of innate defensive actions, emerging new data indicate a prominent role for the PAG in more complex processes, including representing behavioral states and influencing fear learning and memory.
Orchestration of innate and conditioned defensive actions by the periaqueductal gray. Neuropharmacology. 2023 May 1;228:109458. [pdf]
GABAergic CA1 neurons are more stable following context changes than glutamatergic cells
Summary
The CA1 region of the hippocampus contains both glutamatergic pyramidal cells and GABAergic interneurons. Numerous reports have characterized glutamatergic CAMK2A cell activity, showing how these cells respond to environmental changes such as local cue rotation and context re-sizing. Additionally, the long-term stability of spatial encoding and turnover of these cells across days is also well-characterized. In contrast, these classic hippocampal experiments have never been conducted with CA1 GABAergic cells. Here, we use chronic calcium imaging of male and female mice to compare the neural activity of VGAT and CAMK2A cells during exploration of unaltered environments and also during exposure to contexts before and after rotating and changing the length of the context across multiple recording days. Intriguingly, compared to CAMK2A cells, VGAT cells showed decreased remapping induced by environmental changes, such as context rotations and contextual length resizing. However, GABAergic neurons were also less likely than glutamatergic neurons to remain active and exhibit consistent place coding across recording days. Interestingly, despite showing significant spatial remapping across days, GABAergic cells had stable speed encoding between days. Thus, compared to glutamatergic cells, spatial encoding of GABAergic cells is more stable during within-session environmental perturbations, but is less stable across days. These insights may be crucial in accurately modeling the features and constraints of hippocampal dynamics in spatial coding.
Sci Rep. 2022 Jun 20;12(1):10310. [pdf]
Sparse genetically defined neurons refine the canonical role of periaqueductal gray columnar organization
Summary
The periaqueductal gray (PAG) in the midbrain is known to be a central node controlling defensive behaviors. However, the PAG contains diverse cell types that vary in neurochemistry. How these cell types contribute to defense remains unknown. Though prior works showed that broad excitation of the lateral or ventrolateral PAG causes freezing, we found in mice that activation of cholecystokinin-expressing (CCK) cells in this same region elicited flight, which is a completely different action than freezing. Additionally, CCK cells were more active far from threats, while pan-neuronal PAG activity was higher when the mouse was near a threat. Thus, in addition to the anatomical columnar architecture of the PAG, the molecular identity of PAG cells may confer an additional axis of functional organization, revealing unexplored functional heterogeneity
Elife. 2022 Jun 8;11:e77115. doi: 10.7554/eLife.77115. [pdf]
Shared dorsal periaqueductal gray activation patterns during exposure to innate and conditioned threats
Summary
The brainstem dorsal periaqueductal gray (dPAG) has been widely recognized as being a vital node orchestrating the
responses to innate threats. However, it is unknown whether the dPAG displays independent or shared patterns of activation during exposure to innate and conditioned threats. Here, we show that dPAG ensembles encoded not only distance to threat, but also relevant features, such as predator speed and the angle between mouse and threat. Furthermore, dPAG cells accurately encoded numerous defensive behaviors, including freezing, stretch-attend postures, and escape. DPAG cells also displayed a shared representation to encode these features across innate and conditioned threats. These data indicate that a common pathway may be recruited by the dPAG during exposure to a wide variety of threat modalities.
Shared dorsal periaqueductal gray activation patterns during exposure to innate and conditioned threats. J Neurosci. 2021 Apr 21:JN-RM-2450-20. doi:Save 10.1523/JNEUROSCI.2450-20.2021. [pdf]
Dorsal premammillary projection to periaqueductal gray controls escape vigor from innate and conditioned threats
Summary
Escape from threats has paramount importance for survival. However, it is unknown if a single circuit controls escape vigor from innate and conditioned threats. Cholecystokinin (cck)- expressing cells in the hypothalamic dorsal premammillary nucleus (PMd) are necessary for initiating escape from innate threats via a projection to the dorsolateral periaqueductal gray (dlPAG). We now show that in mice PMd-cck cells are activated during escape, but not other defensive behaviors. PMd-cck ensemble activity can also predict future escape. Furthermore, PMd inhibition decreases escape speed from both innate and conditioned threats. Inhibition of the PMd-cck projection to the dlPAG also decreased escape speed. Intriguingly, PMd-cck and dlPAG activity in mice showed higher mutual information during exposure to innate and conditioned threats. In parallel, human
functional magnetic resonance imaging data show that a posterior hypothalamic-to-dlPAG pathway increased activity during exposure to aversive images, indicating that a similar pathway may possibly have a related role in humans. Our data identify the PMd-dlPAG circuit as a central node, controlling escape vigor elicited by both innate and conditioned threats.
Elife. 2021 Sep 1;10:e69178. [pdf]
Dorsal periaqueductal gray ensembles represent approach and avoidance states
Summary
Animals must balance needs to approach threats for risk-assessment and to avoid danger. The dorsal periaqueductal gray (dPAG) controls defensive behaviors, but it is unknown how it represents states associated with threat approach and avoidance. We identified a dPAG threat/avoidance ensemble in mice that showed higher activity far from threats. These cells were also more active during threat avoidance behaviors such as escape and freezing, even though these behaviors have antagonistic motor output. Conversely, the threat approach ensemble was more active during risk-assessment behaviors and near threats. Furthermore, avoidance/approach states were encoded with shared activity patterns across threats. Thus, dPAG ensembles dynamically encode threat approach and avoidance states, providing a flexible mechanism to balance risk-assessment and danger avoidance
Elife. 2021 May 6;10:e64934. [pdf]
Coordination of escape and spatial navigation circuits orchestrates versatile flight from threats
Summary
In the presence of high-intensity danger, humans and other animals attempt to escape from the source of threat. To do so, we cannot run randomly towards any direction away from threat. Instead, we must use our knowledge of the layout of the environment to figure out where are the nearest and most efficient escape routes. Thus, in naturalistic situations, it is common that escapes from threats require a degree of spatial navigation skill. However, in the lab typically escape and navigation are studied in separate experiments by different labs. We reasoned that the little studied dorsal premammillary nucleus in the hypothalamus (PMd) may play a role in such naturalistic escapes because it is interconnected both with spatial navigation regions as well as threat-induced escape nuclei. The two main outputs of the PMd are the anterior medial ventral thalamus (Amv) and the periaqueductal gray (PAG). The Amv and PAG, are, respectively, already known to be important for spatial navigation and escape from threats. Here, we show that activation of the PMd output to the PAG was required for all escapes from threat, indicating this circuit has a general role in promoting flight from threat.
However, intriguingly, activity in the PMd output to the Amv was only recruited in escapes that required spatial navigation. This PMd-amv circuit was not necessary for escapes from threat that just required flight away from threat in a random direction.
These data show that the PMd can create naturalistic escape rom threats by coordinating the activity of spatial navigation circuits (Amv) and flight circuits (via the PMd output to the PAG). This was the first study to investigate the neural basis of such versatile context-specific escapes from threats that require knowledge of the environmental geometry and layout for efficient flight.
Coordination of escape and spatial navigation circuits orchestrates versatile flight from threats. Neuron. 2021 Apr 13:S0896-6273(21)00203-8. [pdf]
Long-Term Characterization of Hippocampal Remapping during Contextual Fear Acquisition and Extinction
Summary
The dorsal hippocampus is necessary for learning that a particular location in an environment is dangerous. Continuous exposure to this dangerous location in the absence of threat gradually decreases the previously acquired fear in a process named fear extinction. Here, we show how hippocampal neural activity is altered across time, during fear acquisition, retrieval and extinction. To study fear acquisition, mice were placed in a long corridor. At one of the ends of the corridor there was a grid that delivered mild footshocks. The mice quickly learn to avoid the shock grid. Across many daily exposures to this environment in the absence of shocks, mice gradually extinguish their fear for the shock grid. To study the role of the hippocampus in this process we recorded the neural activity of hippocampal neurons. This region has cells called ‘place cells’ that are active in specific spatial locations. The location in the environment in which a particular place cell is active is called its ‘place field’. We show that following fear acquisition, there is a concentration of cells with new place fields that appear near the shocking grid. Furthermore, neural activity near the shock grid has higher spatial selectivity. It is as if the hippocampal representation of space became sharper near the threat, because it is particularly important for the mouse to be aware of its proximity to the threat. Strikingly, both of these effects gradually faded away across days during fear extinction. These data show that the hippocampus dynamically represents its representation of space, increasing and decreasing the spatial information content of place cells with ongoing levels of fear behaviors.
Long-Term Characterization of Hippocampal Remapping during Contextual Fear Acquisition and Extinction. J Neurosci. 2020;40(43):8329-8342 [pdf]
Basomedial Amygdala Mediates Top-Down Control of Anxiety and Fear
Summary
Anxiety-related conditions are among the most difficult neuropsychiatric diseases to treat pharmacologically, but respond to cognitive therapies. There has therefore been interest in identifying relevant top-down pathways from cognitive control regions in medial prefrontal cortex (mPFC). Identification of such pathways could contribute to our understanding of the cognitive regulation of affect, and provide pathways for intervention. Previous studies have suggested that dorsal and ventral mPFC subregions exert opposing effects on fear, as do subregions of other structures. However, precise causal targets for top-down connections among these diverse possibilities have not been established. Here we show that the basomedial amygdala (BMA) represents the major target of ventral mPFC in amygdala in mice. Moreover, BMA neurons differentiate safe and aversive environments, and BMA activation decreases fear-related freezing and high- anxiety states. Lastly, we show that the ventral mPFC–BMA projection implements top-down control of anxiety state and learned freezing, both at baseline and in stress-induced anxiety, defining a broadly relevant new top-down behavioural regulation pathway.
Activation of the mPFC-amygdala projection suppresses both innate aversion to danger as well as learned fear-conditioned behaviors. Adhikari et al., Nature, 2015 [pdf]
Diverging Neural Pathways Assemble a Behavioural State from Separable Features in Anxiety
Summary
Behavioural states in mammals, such as the anxious state, are char- acterized by several features that are coordinately regulated by diverse nervous system outputs, ranging from behavioural choice patterns to changes in physiology (in anxiety, exemplified respectively by risk-avoidance and respiratory rate alterations)1,2. Here we investigate if and how defined neural projections arising from a single coordinating brain region in mice could mediate diverse features of anxiety. Integrating behavioural assays, in vivo and in vitro electrophysiology, respiratory physiology and optoge- netics, we identify a surprising new role for the bed nucleus of the stria terminalis (BNST) in the coordinated modulation of diverse anxiety features. First, two BNST subregions were unex- pectedly found to exert opposite effects on the anxious state: oval BNST activity promoted several independent anxious state fea- tures, whereas anterodorsal BNST-associated activity exerted anxiolytic influence for the same features. Notably, we found that three distinct anterodorsal BNST efferent projections—to the lat- eral hypothalamus, parabrachial nucleus and ventral tegmental area—each implemented an independent feature of anxiolysis: reduced risk-avoidance, reduced respiratory rate, and increased positive valence, respectively. Furthermore, selective inhibition of corresponding circuit elements in freely moving mice showed opposing behavioural effects compared with excitation, and in vivo recordings during free behaviour showed native spiking patterns in anterodorsal BNST neurons that differentiated safe and anxio- genic environments. These results demonstrate that distinct BNST subregions exert opposite effects in modulating anxiety, establish separable anxiolytic roles for different anterodorsal BNST projec- tions, and illustrate circuit mechanisms underlying selection of features for the assembly of the anxious state.
The extended amygdala uses different outputs to separately control behavioral and physiological symptoms of anxiety. Kim*, Adhikari* et al., Nature, 2013 [pdf]
Single Units in the Medial Prefrontal Cortex with Anxiety-Related Firing Patterns Are Preferentially Influenced by Ventral Hippocampal Activity
Summary
The medial prefrontal cortex (mPFC) and ventral hippocampus (vHPC) functionally interact during innate anxiety tasks. To explore the consequences of this interaction, we examined task-related firing of single units from the mPFC of mice exploring stan- dard and modified versions of the elevated plus maze (EPM), an innate anxiety paradigm. Hippocampal local field potentials (LFPs) were simultaneously monitored. The population of mPFC units distin- guished between safe and aversive locations within the maze, regardless of the nature of the anxiogenic stimulus. Strikingly, mPFC units with stronger task- related activity were more strongly coupled to theta-frequency activity in the vHPC LFP. Lastly, task-related activity was inversely correlated with behavioral measures of anxiety. These results clarify the role of the vHPC-mPFC circuit in innate anxiety and underscore how specific inputs may be involved in the generation of behaviorally relevant neural activity within the mPFC.
The mPFC may use hippocampal input to differentiate safe and dangerous locations. Adhikari et al., Neuron, 2011 [pdf]
Synchronized Activity between the Ventral Hippocampus and the Medial Prefrontal Cortex during Anxiety
Summary
The hippocampus has been studied for decades due to its central role in the acquisition and storage of memories. These experiments were done in the dorsal part of the hippocampus. However, the ventral region of the hippocampus (vHPC) has been much less studied. The vHPC unlike its dorsal counter- part, is required for anxiety-like behavior. The means by which it acts are unknown. We hypothesized that the vHPC synchronizes with downstream targets that influence anxiety, such as the medial prefrontal cortex (mPFC). To test this hypothesis, we recorded mPFC and vHPC neural activity in mice exposed to two anxiogenic arenas. Theta- frequency (4-12 Hz) activity in the mPFC and ventral, but not dorsal, hippocampus was highly correlated at base- line, and this correlation increased in both anxiogenic environments. Increases in mPFC theta power predicted avoidance of the aversive compartments of each arena and were larger in serotonin 1A receptor knockout mice, a genetic model of increased anxiety-like behavior. These results suggest that the vHPC becomes more synchronized with the mPFC, potentially creating higher anxiety states.
Synchrony of neural activity between the ventral hippocampus and the medial prefrontal cortex (mPFC) predicts avoidance of risk. Adhikari et al., Neuron, 2010 [pdf]
Synchronized Activity between the Ventral Hippocampus and the Medial Prefrontal Cortex during Anxiety
Summary
The hippocampus has been studied for decades due to its central role in the acquisition and storage of memories. These experiments were done in the dorsal part of the hippocampus. However, the ventral region of the hippocampus (vHPC) has been much less studied. The vHPC unlike its dorsal counter- part, is required for anxiety-like behavior. The means by which it acts are unknown. We hypothesized that the vHPC synchronizes with downstream targets that influence anxiety, such as the medial prefrontal cortex (mPFC). To test this hypothesis, we recorded mPFC and vHPC neural activity in mice exposed to two anxiogenic arenas. Theta- frequency (4-12 Hz) activity in the mPFC and ventral, but not dorsal, hippocampus was highly correlated at base- line, and this correlation increased in both anxiogenic environments. Increases in mPFC theta power predicted avoidance of the aversive compartments of each arena and were larger in serotonin 1A receptor knockout mice, a genetic model of increased anxiety-like behavior. These results suggest that the vHPC becomes more synchronized with the mPFC, potentially creating higher anxiety states.
Synchrony of neural activity between the ventral hippocampus and the medial prefrontal cortex (mPFC) predicts avoidance of risk. Adhikari et al., Neuron, 2010 [pdf]